
Analysis of ANVISA's Regulatory Sandbox Proposal for Medicinal Cannabis Research
Technical, legal, and regulatory analysis of the regulatory sandbox proposed by Anvisa for medicinal cannabis research, by Marcelo Polacow Bisson and Priscila Gava Mazzola. (FCF-UNICAMP; GTT de Cannabis Medicinal CRFSP)
Published at 02/01/2026The National Health Surveillance Agency (ANVISA) proposed the establishment of a Regulatory Experimental Environment (Regulatory Sandbox) for research with Cannabis sativa for medicinal purposes. The initiative arises in the context of a growing demand for cannabinoid-based therapies and the need to overcome historical barriers that limit scientific research in Brazil. This analysis evaluates the proposal, weighing challenges, constraints, and opportunities for companies, universities, and the development of a national ecosystem of innovation in medicinal cannabis.
Technical Analysis
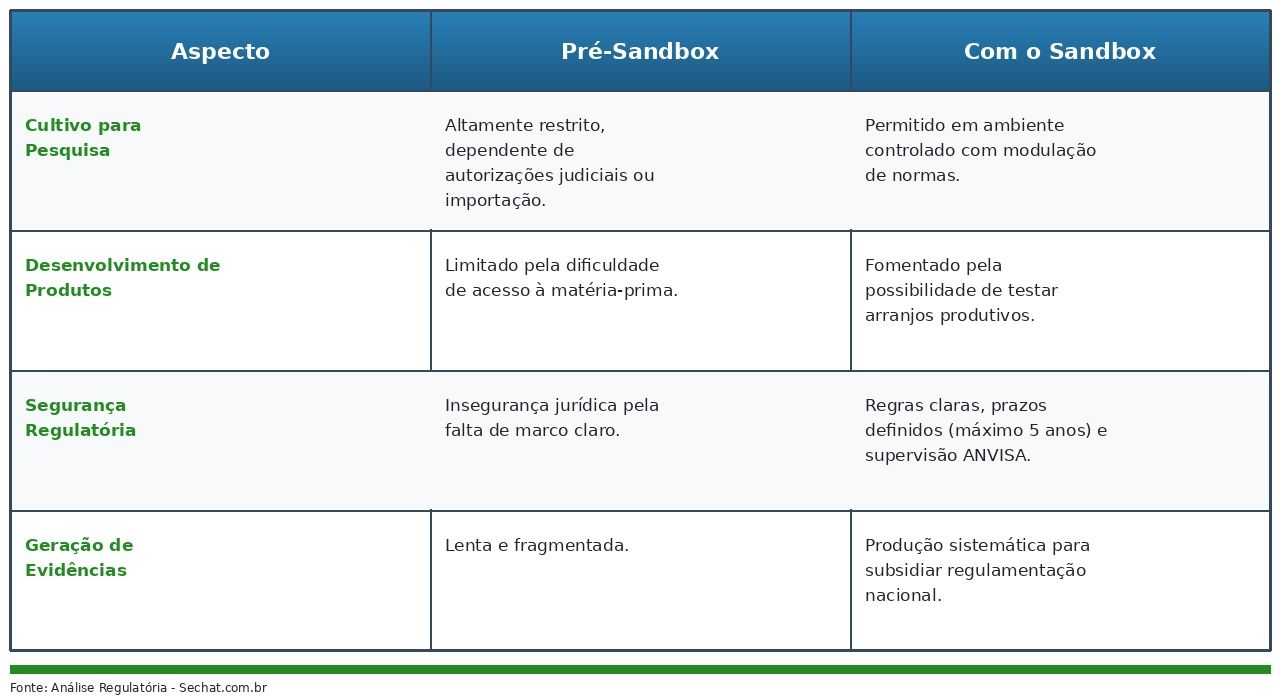
The guarantee of quality, safety, and efficacy of products is essential, depending on rigorous technical standardization throughout the production chain. The absence of clear guidelines results in product heterogeneity, hindering clinical research [1].
ANVISA's Sandbox addresses this issue by creating a controlled environment for "supervised testing of technical, productive, and operational arrangements on a small scale" (Art. 4). Within this environment, it will be possible to develop standard operating procedures, from controlling the origin of the plant material to characterizing the cannabinoid profile.
The requirement for a Technical Responsible and production records are essential for generating robust data and regulatory evidence.
Legal and Regulatory Analysis
The Sandbox represents significant innovation. Established by Complementary Law No. 182/2021, it allows for the temporary suspension of certain existing regulations to test new technologies under regulatory supervision.
ANVISA proposes "regulatory modulation" of regulations such as RDC No. 327/2019, overcoming "critical obstacles" such as slowness and overlapping demands [2]. The nature is explicitly "experimental, transitory, and temporary," not generating "acquired rights or legitimate expectations of continuity" (Art. 3 and 22).
By prohibiting commercialization and industrial production, ANVISA balances innovation promotion with public health protection, aligning with the STJ decision on regulating cultivation for medicinal and research purposes [4].
Challenges and Opportunities
The main challenge is to ensure that the selection and monitoring process is agile, transparent, and based on solid technical criteria. The dependence on importing inputs and the difficulty of interinstitutional collaboration [2] need to be addressed in the call for proposals.
The opportunities are significant. For universities and research centers, the Sandbox allows for conducting agronomic, genetic, pharmacological, and clinical studies with autonomy and legal certainty. For companies and startups, it reduces entry barriers and allows for product validation with lower regulatory costs.
The collaboration between these sectors can accelerate innovation and position Brazil as a center of excellence in medicinal cannabis.
Points of Attention
Despite its innovative potential, the regulatory sandbox presents limitations that require caution. Depending on its implementation, the flexibility may be more formal than effective, reproducing requirements similar to those of conventional sanitary registration and making the experimental environment costly and less accessible.
International experiences show that experimental regulatory environments tend to favor already structured institutions, with greater financial capacity and specialized regulatory teams. Without inclusive criteria and support mechanisms, there is a risk of excluding public universities, emerging groups, and scientific startups.
In addition, there is a mismatch between the temporary nature of the sandbox and the time required to produce robust scientific evidence, as well as uncertainty about the incorporation, into permanent regulations, of the data generated. Finally, it is essential to recognize that the sandbox is a transitional instrument and does not replace the construction of a structured regulatory framework for medicinal cannabis in Brazil.
Conclusion
ANVISA's proposal to establish a Regulatory Sandbox for medicinal cannabis research is a strategic and welcome initiative, demonstrating a modern and pragmatic stance by the regulatory agency. By creating a controlled flexibility environment, the measure has the potential to unlock research and technological development in the sector, overcoming years of stagnation.
Success will depend on careful implementation that ensures agility and transparency, and continuous commitment from regulators, researchers, companies, and civil society to turn potential into reality, consolidating a regulatory framework based on solid scientific evidence that meets the needs of public health.
References
[1] DA COSTA, S. P. Medicinal cannabis: pharmaceutical and regulatory challenges. ARACÊ, v. 7, n. 7, p. 37901–37909, 2025.
[2] NUNES, T.; PERRI, A. Experts from 31 institutions propose regulatory framework for medicinal cannabis. Jornal da Unicamp, Aug 18, 2025.
[3] SOUZA, M. R.; HENRIQUES, A. T.; LIMBERGER, R. P. Medical cannabis regulation: overview of models with emphasis on Brazil. Journal of Cannabis Research, v. 4, art. 33, 2022.
[4] SUPERIOR TRIBUNAL DE JUSTIÇA. IAC 16. Brasília, DF, Nov 2024.
Priscila Gava Mazzola*, is a professor at the Faculty of Pharmaceutical Sciences of the State University of Campinas (FCF/Unicamp). Graduated from USP/SP, with a Ph.D. in pharmaceutical biochemistry technology and enhanced skills at MIT. Dr. Priscila is also a specialist in topical and transdermal medications, using natural (including residues) and synthetic actives in her work. She is currently exploring the therapeutic powers of medicinal cannabis, developing new medications to expand the national therapeutic arsenal.