Results
Filtros

RDC 327 vote postponed at Anvisa; ABIQUIFI evaluates new deadline as "legitimate tool"
Brazilian Association of Pharmaceutical Inputs Industry trusts that director Thiago Campos' request for review will result in a more thorough analysis, maintaining the expectation of regulatory framework completion in January 2026

Maceió to host open class on medical cannabis this Friday (07)
Initiative complies with State Law 8,754/22 and aims to prepare professionals to prescribe cannabis safely and legally

Cannabis importation in SP grows 6.2% in the 1st semester of 2025
Anvisa authorizations for importing cannabidiol in São Paulo increased by 6.2% between January and July 2025, consolidating the state as a leader in requests and reflecting the expanded access through the SUS

Ways to Access Cannabis Products in Brazil
01/18/2023Understand the step-by-step process to acquire plant derivatives for medicinal purposes

2026 reserves crucial legislative advances for medical cannabis in Brazil
Resumption of Anvisa review and STJ deadline for cultivation define the sector's direction in the first quarter of the year

Cannabis Connection 2025 takes place in São Paulo in a decisive year for the cannabis market
The event brings together experts, companies, and authorities to discuss regulatory advances, research, and innovation in cannabis-based therapies, connecting the entire public and private sector

Globo Rural covers medicinal cannabis and industrial hemp in special report
Debates on medicinal cannabis continue deeply at Cannabis Connection 2025 in November and at the Brazilian Medicinal Cannabis Congress in May 2026, both in São Paulo.

Professor Advocates for Inclusion of Physical Therapists in Cannabis-Based Treatment
COFFITO Ruling No. 735/2024 authorizes physical therapists with specific training to prescribe medications and supplies; however, Anvisa recognizes only doctors and dentists as prescribers of cannabis for medicinal purposes

SUS and Health Insurance Plans
01/01/2024Learn How to Access Cannabis Products Through the Unified Health System and Understand the Obligations of Health Insurance Providers

RDC 327 review rapporteur bids farewell to Anvisa this Friday (19).
Rômison Rodrigues Mota leaves the position after a request for review delays the norm's vote; the topic will return to the regulatory agenda for 2026-2027

Anvisa warns of deadline for comments on agenda including review of RDC 327/2019
Submission of registrations, videos, and requests for confidentiality ends at 11:59 pm this Friday (5); item revising rules for cannabis products will be defined at the Dicol meeting on December 10

Anvisa incorporates RDC 327 rules into Ordinance 344 and formalizes veterinary prescription of cannabis
11/26/2025Anvisa's update "mirrors" the rules for controlling cannabis products directly in the list of controlled substances and defines classification for industrial inputs
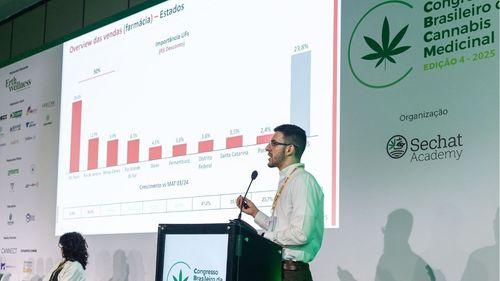
Cannabidiol grows over 70% in the pharmacy channel; Anvisa's RDC 327 review generates optimism in the sector
According to Filipe Campos, from Close-Up International, the market could expand even further with rule updates and greater medical adherence

RDC-327 and Cannabis Research: Lawyer Explains Proposal to Extend Deadline for Clinical Studies
On Deusa Cast, lawyer Larissa Meneghel details how the five-year deadline set forth in RDC-327 for clinical cannabis research works and comments on the renewal proposal under review by Anvisa

Anvisa may vote on maintaining or removing the agenda item for the review of RDC 327/2019, which regulates medicinal cannabis products
The item revising RDC 327/2019 reaches a decisive week at Anvisa, and the sector awaits to know if the topic will proceed to a vote or be postponed once again

Anvisa to decide today the future of RDC 327 in a meeting that could redefine rules for cannabis products in Brazil
Norm that regulates manufacturing, importation, prescription, and inspection may be entirely revoked and replaced by a new regulatory framework; decision will be made at the Collegiate Board meeting.

Anvisa publishes proposal to update RDC 327 redefining rules for cannabis products in Brazil
Draft presents new regulatory framework, tightens requirements for Sanitary Authorization, and prohibits manipulation of cannabis derivatives

"Legally consolidates what had already been built," says veterinarian about new Anvisa regulation
Caroline Campagnone views RDC 999/2025 favorably, allowing veterinarians authorized by CFMV to prescribe medical cannabis

“It is important that the compounding pharmacy has access to all cannabis inputs" argues Marco Fiaschetti, from Anfarmag
With the revision of RDC 327/2019, compounding pharmacies gain space in the discussion on medicinal cannabis

Anvisa Collegiate Board has new composition defined after director's departure
Marcelo Mario Matos Moreira takes over as substitute in the Fourth Board, filling the vacancy of the former rapporteur of the review of RDC 327