Government asks STJ for additional 180 days to deliver regulation on cannabis cultivation
Ministry of Health and Anvisa claim technical complexity and propose a new schedule that extends until March 2026 to define the rules
Published on 10/01/2025

Palácio do Planalto. Ricardo Stuckert/PR
The Federal Government requested the Superior Court of Justice (STJ) a 180-day extension to publish the regulation that defines the medicinal cannabis regulation in Brazil. The request was made on Tuesday (9/30) to Minister Regina Helena Costa, the case's rapporteur.
The original deadline for the Union and the National Health Surveillance Agency (Anvisa) to publish the normative act, according to the action plan, ended on September 30. The STJ's decision required the creation of rules for the importation of seeds, planting, and commercialization of the plant for exclusively medicinal purposes.
The Ministry of Health (MS) and Anvisa argue that the extension of the deadline is necessary due to the high technical and regulatory complexity of the subject. According to the agencies, the process requires an internal restructuring at the agency.
In addition, it is necessary to carry out a thorough Regulatory Impact Analysis (RIA) and ensure social participation through public consultation. Therefore, the new schedule extends the stages of medicinal cannabis regulation until March 2026.
New schedule for regulation
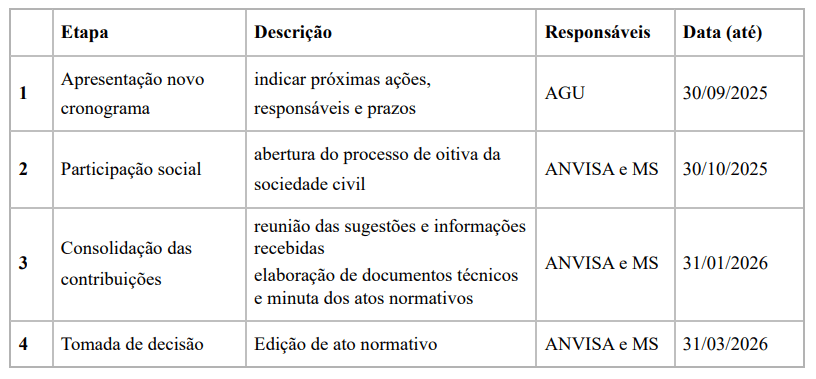
The proposal sent to the STJ details a new six-month plan that includes the hearing of civil society for popular participation. Subsequently, the received contributions will be analyzed and compiled.
After this phase, the documents and the draft of the normative act will be prepared to finally publish the regulation norm. The original plan had been developed with the participation of experts, patient associations, and scientific institutions.
Current plan has unfulfilled stages
After the approval of the initial action plan by the STJ in June 2025, several administrative and regulatory stages remained pending. The government justified that it was not possible to carry out the actions foreseen in items 6 to 9 of the previous schedule.
The unfulfilled stages, which had a deadline until September 2025, were crucial for the regulation of medicinal cannabis and included:
- Consolidation of contributions and final drafting of the Ordinance draft;
- Preparation of a technical note for the final version of the Ordinance (MS);
- Legal analysis of the draft and preparation of an opinion (AGU);
- Final decision and approval for the publication of the Ordinance (MS);
- Voting on a new Collegiate Board Resolution (Anvisa) to adjust the rules for the control of the Cannabis sativa L. plant with low THC content.










