Results
Filtros

CFF advocates for magistral manipulation amid changes in Cannabis regulation
Entity reinforces that personalized preparations are essential when industrialized products do not meet clinical needs

Anvisa approves new framework for herbal medicines in Brazil
New regulation simplifies registration and expands the use of biodiversity in herbal medicines, as soon as cannabis products are classified as medicines, they must comply with these standards.

Anvisa approves new cannabis-based product in Brazil
Pharmaceutical company Ephar has obtained registration for its Cannabidiol in two concentrations, 50 mg/mL and 100 mg/mL, which will be marketed in a low THC content drop solution

CRF-SP warns of risk of setback and legal uncertainty in the review of the Cannabis regulation
According to Marcelo Polacow Bisson, excluding pharmacies' manipulation leads to lack of assistance; Anvisa's proposed text on the Cannabis regulation is also criticized for bypassing procedural rites

The quality of cannabis products produced by the industry in Brazil
How the pharmaceutical industry ensures quality and safety in cannabis-based products sold in Brazil

“Special Moment for the Industry”: Executive Foresees Advancements with Anvisa's Review
Gustavo Palhares believes that regulatory review could streamline prescription, expand access, and raise technical requirements in the medical cannabis sector

Market expansion drives opening of Anvisa-regulated cannabis pharmacy in Paraná
Curitiba welcomes its first exclusive medicinal cannabis pharmacy, in a market that grew from R$ 38.5 million in 2022 to R$ 237.8 million in 2025, while Paraná accounts for only 2.4% of sales, indicating great potential in the state with 11 million inhabitants

43rd CIOSP discusses the future of cannabinoid therapies in Dentistry
Lecture led by specialist will address scientific updates, clinical use, and regulation of cannabinoid therapies in Dentistry during the event in São Paulo

Traveling with Medical Cannabis at the End of the Year: What Patients Need to Know to Avoid Issues
Planning to travel at the end of the year and using medical cannabis? Learn about the required documents, how to transport the medication, and what precautions to take in Brazil and abroad

Anvisa approves two cannabis-based products for pharmacies
Pharmaceutical company Farmausa Life Science has obtained registration for its Cannabidiol in two concentrations, 20 mg/mL and 100 mg/mL, with low THC content

Anvisa approves new cannabis-based product in Brazil
Cannten's extract with 71.33 mg/mL expands medicinal treatment options in the country and receives authorization for commercialization

Anvisa approves new pharmaceutical product based on cannabis in Brazil
Endogen's extract expands the portfolio of options for medicinal treatments and already has sanitary authorization for commercialization

The Year of Cannabis: trends and projections for the market in 2026
Financial consolidation, cultivation regulation, and strengthening of events mark the sector's prospects for the next year

Cannabis cultivation and regulatory review take center stage in the 2026–2027 Regulatory Agenda
Approved document defines 161 priority topics for the next two years, including national cultivation of low-THC cannabis

Anvisa blocks advertising of pharmaceutical cannabis products on digital platforms
Preventive action involves imported and national items and reinforces rules for restricted disclosure to doctors and pharmacists

Last call for the largest medicinal cannabis event in Latin America
With limited spots available, Cannabis Connection 2025 will host the launch of the 5th Sechat Cannabis Guide and discussions on regulation, cultivation, and market

Univali authorized by Anvisa to perform analyses of Cannabis products
New authorization expands university's scope in Reblas and reinforces quality control of plant derivatives in the national market
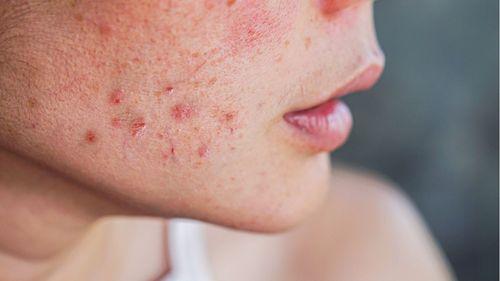
Cannabis in the Treatment of Skin Diseases: 3 Science-Backed Benefits
Studies show efficacy of cannabinoids in controlling inflammation, acne, and itching, while Anvisa evaluates expanding regulations for topical products

With inspirations ranging from American bluegrass to Gabriel García Márquez literature, entrepreneur points to "mirror" market for Brazil
The Australian medicinal cannabis market is consolidating as an international reference and inspiring discussions in Brazil

Anvisa Regulatory Agenda 2026 - 2027 opens space for society suggestions, including on cannabis
Population and regulated sectors have the opportunity to indicate topics of interest for the next biennium