Reblas Network includes cannabis product analysis; Brazil has 16 accredited laboratories
Published by Anvisa, the new resolution expands the scope of the network of analytical laboratories and reinforces the quality control of cannabis-based products in the country
Published on 09/11/2025

The measure was confirmed by Resolution No. 3,459, published on September 8 in the Official Gazette. Image: Canva pro
Public or private analytical laboratories accredited by the National Health Surveillance Agency (Anvisa) are officially authorized to perform analyses of cannabis products. The measure was confirmed by Resolution No. 3,459, published on September 8 in the Official Gazette.
The resolution modifies the scope of the Analytical Quality Center Ltd., a member of the Brazilian Network of Analytical Laboratories in Health (Reblas), officially including the category of cannabis products.
As a result, the laboratory now offers quality control services for products derived from the plant, according to Art. 5 of Resolution RDC No. 928/2024:
“Art. 5. Analytical laboratories providing services that perform quality control tests on batches of finished products must be accredited in Reblas in scopes corresponding to the respective categories of analyzed products.”
16 laboratories are already accredited
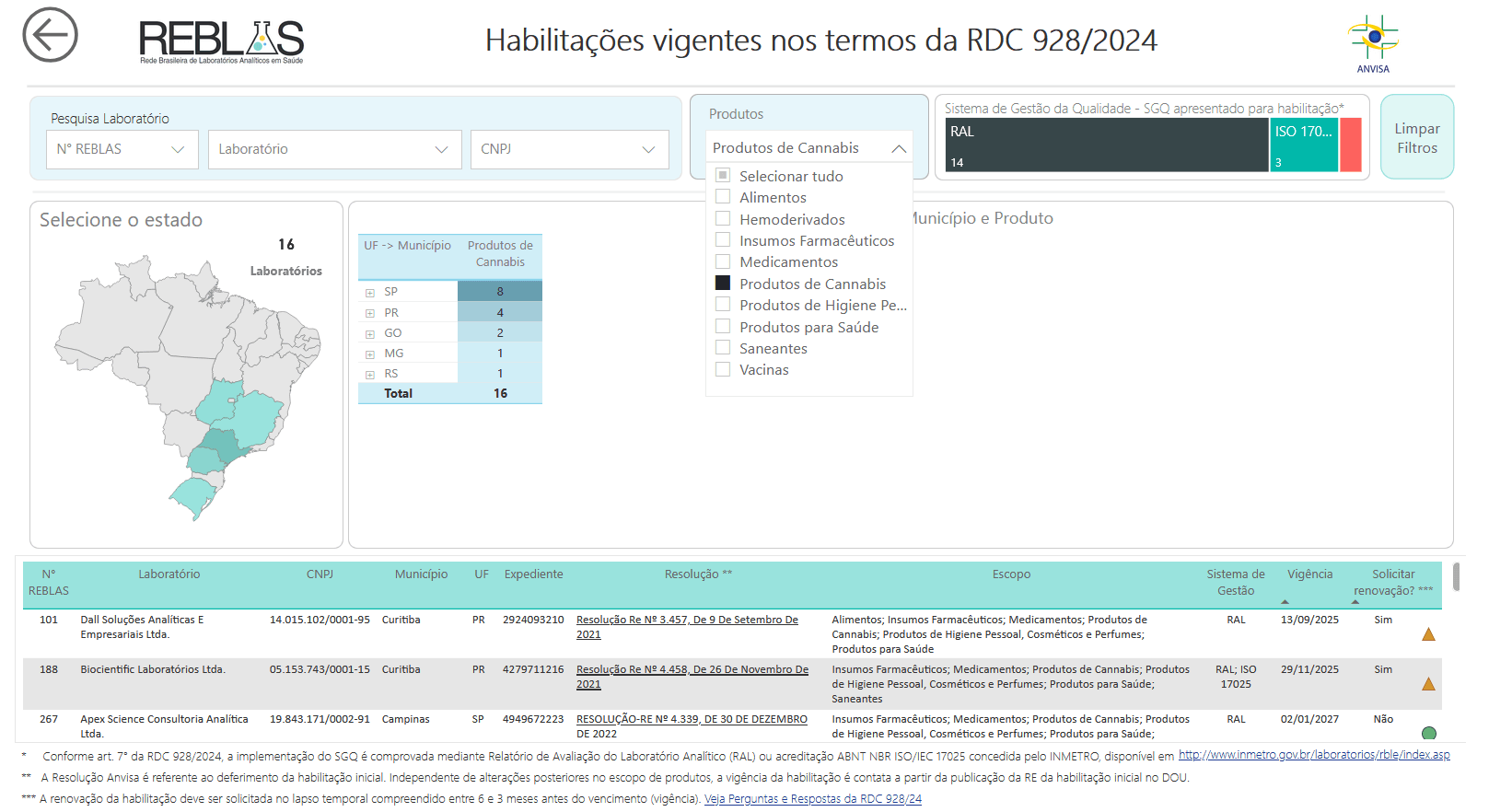
Reblas brings together public and private analytical laboratories accredited by Anvisa to offer sanitary services of quality, safety, reliability, and traceability.
Currently, 16 laboratories are already accredited for the analysis of cannabis products. The state of São Paulo concentrates half of them, followed by Paraná (4), Goiás (2), Minas Gerais (1), and Rio Grande do Sul (1). All accreditations were granted by resolutions of Anvisa.
The scientific director of Sechat, Dr. Pedro Pierro, highlighted the relevance of the measure. “The possibility of ensuring the quality of cannabis-based products through RDC 660, with the support of Reblas laboratories, is essential for doctors and patients.”
An example cited is Carmen's Medicinals, which in 2024 obtained validation attesting that its oils have a high degree of purity and quality. According to the company, this provides security to doctors and patients that they are prescribing and consuming products that meet the most rigorous standards established by Anvisa.
Ricardo Pettená, the company's executive director, emphasizes that the investment in quality is directly reflected in the final product. “Having the Reblas certificate conveys credibility and security to the medical community when prescribing,” he says.
For him, the certification also helps differentiate the “wheat from the chaff,” encouraging the market to adapt and offer products from RDC 660 with standards equal to or even higher than those of RDC 327. “Previously, we only worked with the COA certificate, which, we understand, does not have the same credibility with the market and Anvisa. Reblas highlights which companies truly have a commitment to quality and which do not,” he concludes.








